i-Particles®
Driven by quality, safety and innovation
i-Particles®, a versatile platform driven by safety
Allowing targeted release of vectorised active compounds and sustained drug release over a period of time, those carrier systems may be beneficial in various therapeutic situations by increasing the therapeutic effect while lowering the toxicological concerns about overdose.
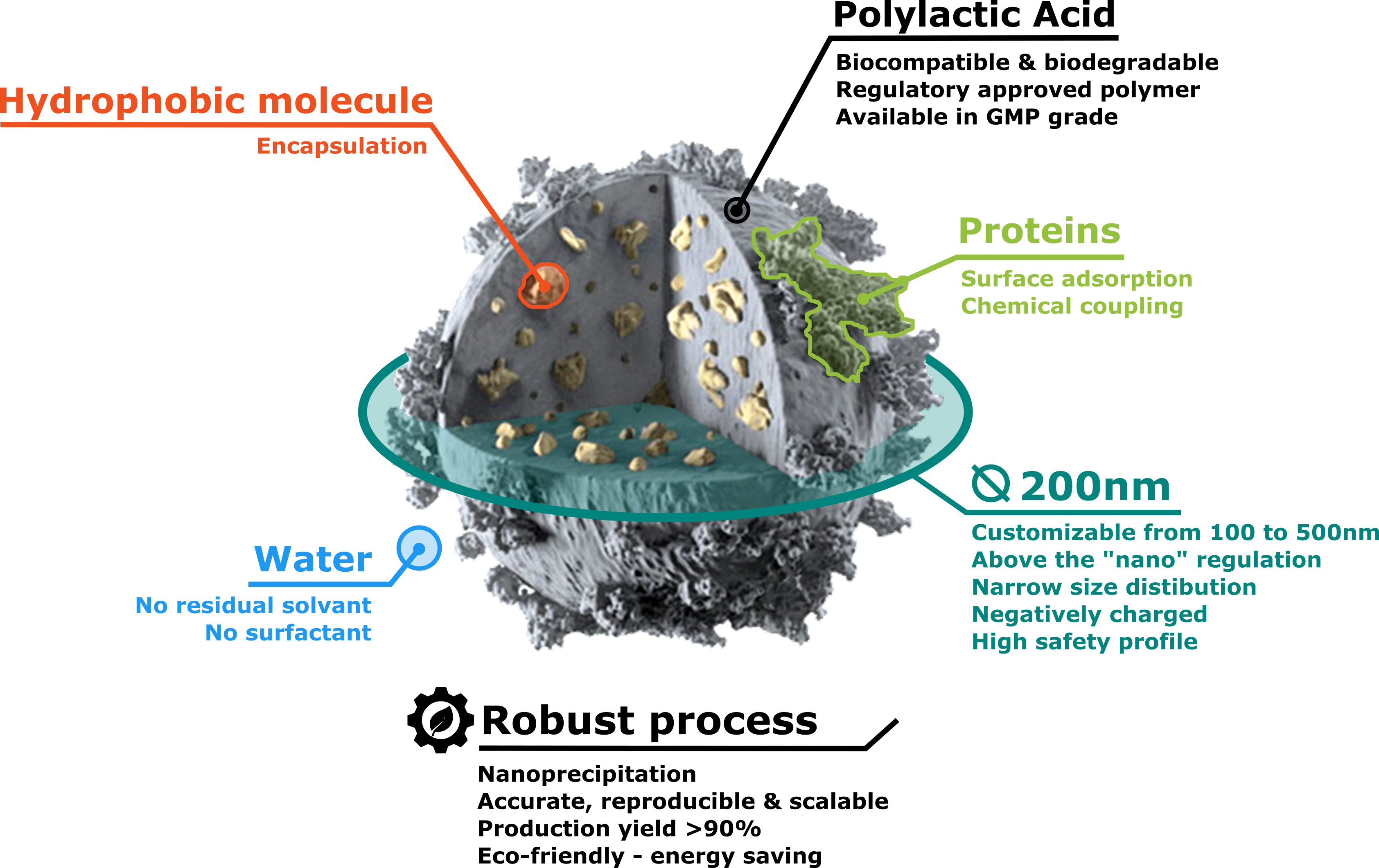
Developed by Adjuvatis to overstep pharmaceutical challenges, i-Particles® technology was born from a safe-by-design concept. Using PLA, a biocompatible and biodegradable polymer already approved by regulatory agencies, particle suspension is only made of polymer in water, meaning no residual solvent and no surfactant. With an average size of 200 nm, our innovative vector is not subjected to the nanomaterial regulation.
Regarding industrial concerns, Adjuvatis has reached the highest level related to process robustness. Our one-step and stress-free synthesis method induces no drug degradation and leads to high production yields and reproducible batches. Easily scalable and adaptable to an automatic continuous process, Adjuvatis can produce from milliliter to liters.
Overstep pharmaceutical development barriers
Technical requirements
Solubilizing hydrophobic drugs
Protecting and stabilizing drugs
Targeting delivery to specific organs/cells
Delivering sustainably
Detoxifying and reducing risk profile
Strategic development
Increasing drug efficacy
Increasing cost-effectiveness
Providing patient-friendly dosage forms
Extending products life cycle (new means of delivery)
Exploring new routes of administration
Your priviliged partner for industrial development
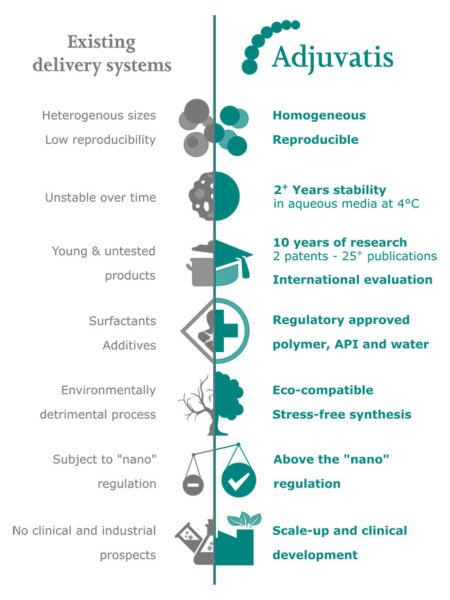
Adjuvatis technology fills the gap of most carrier system drawbacks to reach clinical and industrial developments.
i-LipoP®, the next standard of delivery system
mRNA-loaded carrier systems have gained huge interest after their first use in Covid-19 vaccines. Working on such technology for a few years, Adjuvatis finally commercialized i-LipoP® for nucleic acid vectorization.
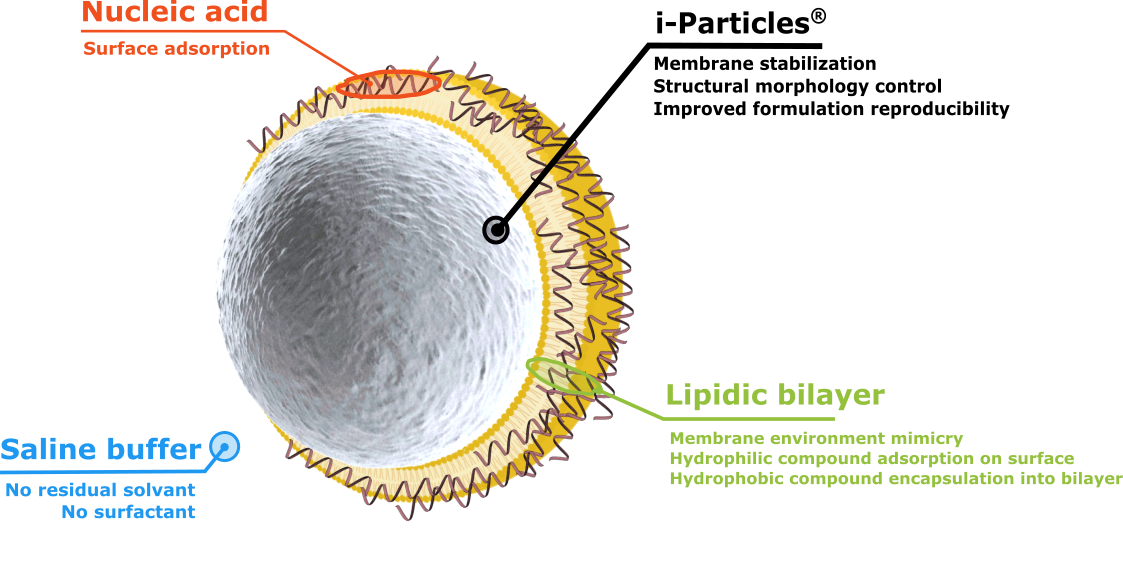
Our second technology, i-LipoP® is composed of i-Particles® in the core of the carrier system acting as a solid spherical support and enabling stabilization of the membrane, controling the structural morphology and improving reproducibility of formulations.
A lipidic bilayer covers the whole surface of the carrier resulting in lipoparticles called i-LipoP®. Mimicrying a membrane environment, hydrophobic compounds may be entrapped into the bilayer while hydrophilic ones are adsorbed on the surface, making possible the vectorization of nucleic acids such as mRNA.
