Following 20 years of development and pre-clinical evaluation, i-Particles® have already proven efficacy in several fields as vaccines, drug delivery and diagnostics while i-LipoP® compete for the next standard of carrier systems. Through an effective research policy, Adjuvatis is involved in many collaborative projects, constantly extending i-Particles® fields of application.
Vaccine Adjuvant
Discover our innovative vaccine adjuvants
Drug Delivery
Find the best way to potentiate your active ingredient
Diagnostics
Know more about the antigen detection
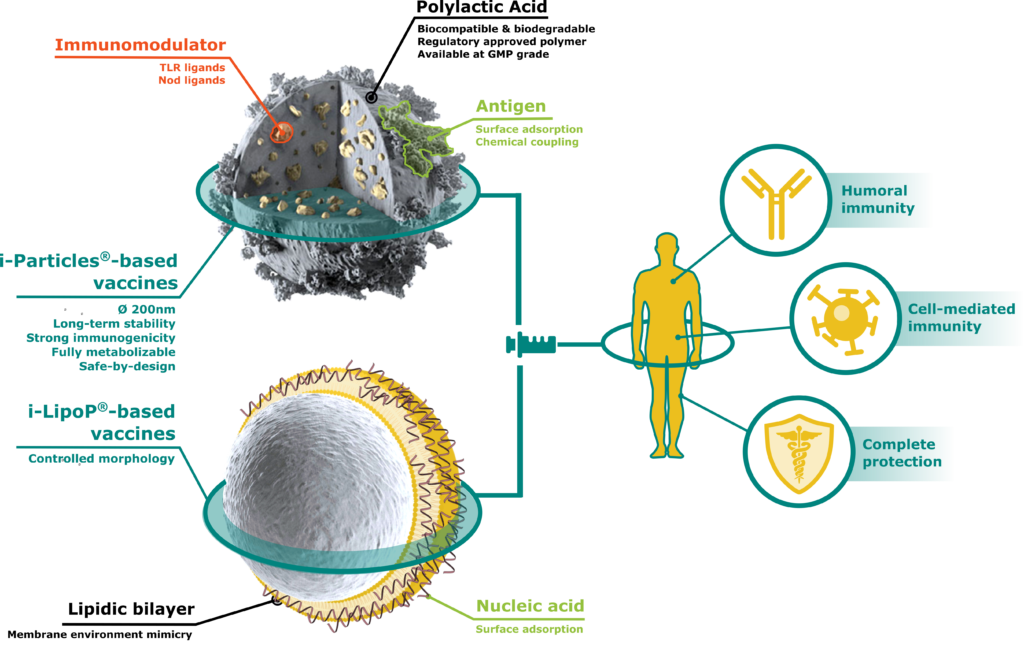
i-Particles® and i-LipoP®, new class of adjuvants
Either alone or encapsulating immunogenic molecules, our technologies are able to greatly stimulate humoral and/or cell-mediated responses of vectorized antigens and increase antigen stability.
The key to unlock the full potential of your active ingredient
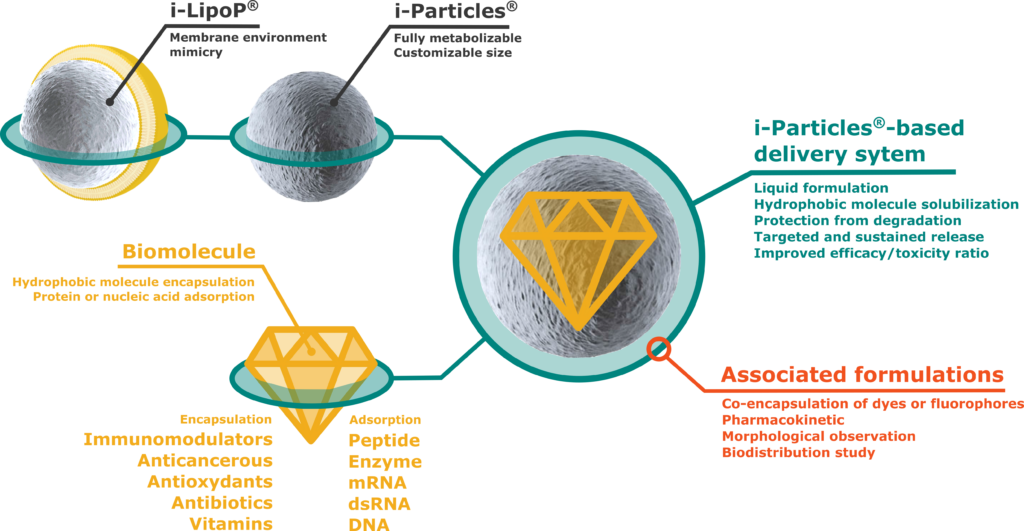
Through an experience with more than 50 biomolecules, Adjuvatis is able to predict molecule formulation parameters and efficiency based on physico-chemical properties of the active ingredient. Once optimized, i-Particles® and i-LipoP® formulations are controlled for long storage stability.
Additionally to the formulation service, a biodistibution study can be performed thanks to co-encapsulation with a fluorophore. From organ scale to body scale, Adjuvatis is able to follow the pathway of i-Particles® and i-LipoP®.
Enhance your antigen detection with plain or dyed i-Particles®
Formulating your antibody or ligand with i-Particles® is the key to reveal antigen or receptor interactions with a biodegradable platform for lateral flow tests, agglutination tests or bead-ELISA.
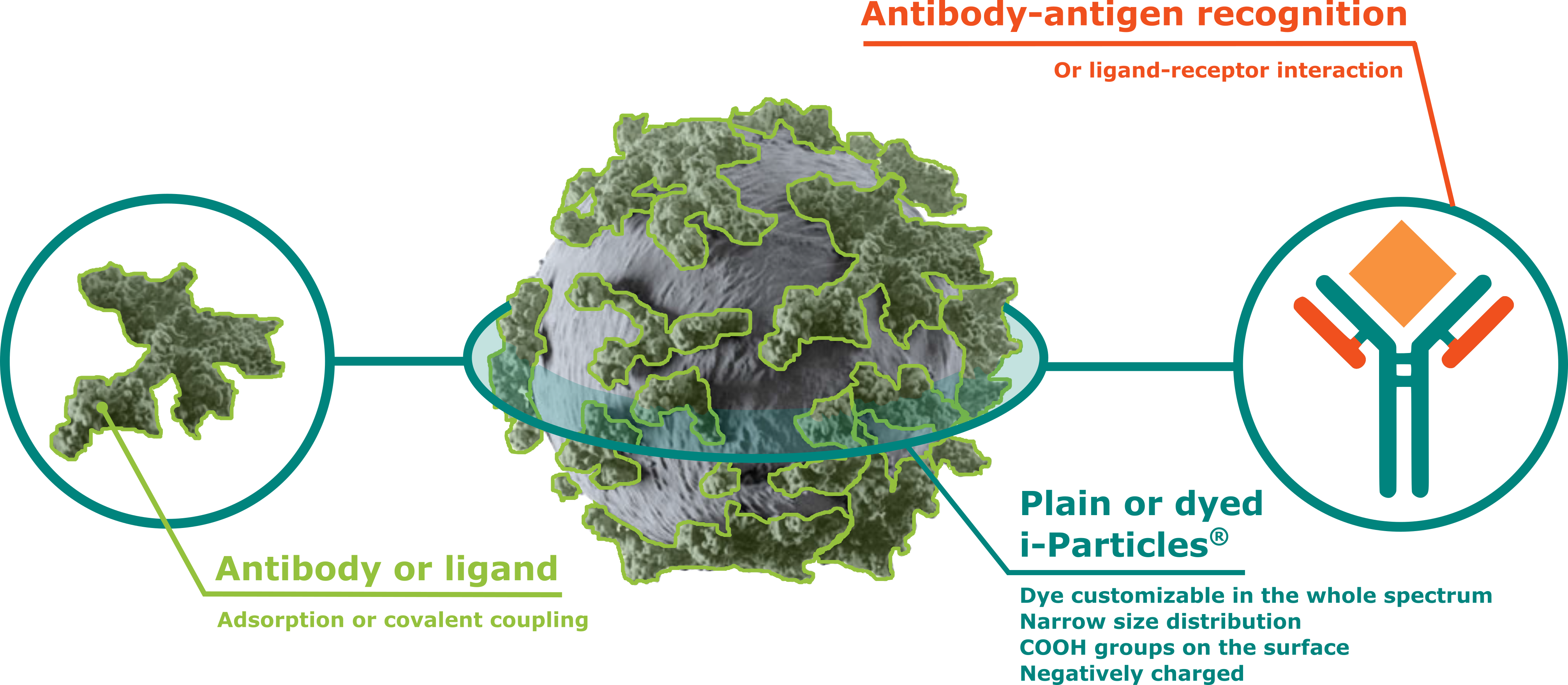
Adjuvatis provides a new class of products based on functionnalized dyed or plain i-Particles® for diagnostic purposes. Thanks to its biodegradable polymer, develop your own eco-compatible tool for high antigen recognition. Entirely customizable, select the best dye or diameter of particles.
Have a look on most relevant published articles
Protective immunity
Jawinski K et al., Recombinant Haemagglutinin Derived From the Ciliated Protozoan Tetrahymena thermophila Is Protective Against Influenza Infection. Front Immunol. 2019. 10:2661.
Adjuvanted i-Particles with immune modulators
Lamrayah, M. et al., Molecular modelling of TLR agonist Pam3CSK4 entrapment in PLA nanoparticles as a tool to explain loading efficiency and functionality. Int J Pharm 568, 118569, 2019.
Gutjahr, A., et al., Cutting Edge: A Dual TLR2 and TLR7 Ligand Induces Highly Potent Humoral and Cell-Mediated Immune Responses. J Immunol, 2017. 198(11): p. 4205-4209.
Pavot, V., et al., Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials, 2016. 75: p. 327-39.
Pavot, V., et al., Cutting edge: New chimeric NOD2/TLR2 adjuvant drastically increases vaccine immunogenicity. J Immunol, 2014. 193(12): p. 5781-5.
Pavot V, et al. Encapsulation of Nod1 and Nod2 receptor ligands into poly(lactic acid) nanoparticles potentiates their immune properties. J Control Release. 2013 Apr 10;167(1):60-7
mRNA-based vaccine
Ayad, C. et al., LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro mRNA Transfection Compared to Liposomes. Pharmaceutics 13, 2021.
Coolen et al. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials. 2019. 195:23-37
Comparison with other adjuvants
Guillon, C., et al., Formulation of HIV-1 Tat and p24 antigens by PLA nanoparticles or MF59 impacts the breadth, but not the magnitude, of serum and faecal antibody responses in rabbits. Vaccine, 2007. 25(43): p. 7491-501.
Skin immunization
Levin, C., et al., Critical role for skin-derived migratory DCs and Langerhans cells in TFH and GC responses after intradermal immunization. J Invest Dermatol, 2017.
Rancan, F., et al., Particle-based transcutaneous administration of HIV-1 p24 protein to human skin explants and targeting of epidermal antigen presenting cells. J Control Release, 2014. 176: p. 115-22.
Long lasting humoral immunity
Ataman-Onal Y, et al . Surfactant-free anionic PLA nanoparticles coated with HIV-1 p24 protein induced enhanced cellular and humoral immune responses in various animal models. J Control Release. 2006 May 15;112(2):175-85.
Lamalle-Bernard D et al, Coadsorption of HIV-1 p24 and gp120 proteins to surfactant-free anionic PLA nanoparticles preserves antigenicity and immunogenicity. J Control Release. 2006 Sep 28;115(1):57-67.
i-Particles biodistribution
Rességuier J et al. Biodistribution of surfactant-free poly(lactic-acid) nanoparticles and uptake by endothelial cells and phagocytes in zebrafish: Evidence for endothelium to macrophage transfer. J Control Release. 2021 Mar 10;331 :228-245.
Rességuier J et al, Specific and Efficient Uptake of Surfactant-Free Poly(Lactic Acid) Nanovaccine Vehicles by Mucosal Dendritic Cells in Adult Zebrafish after Bath Immersion. Front Immunol. 2017 Feb 27;8:190
Targeted delivery
Da Costa, D. et al., Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J Nanobiotechnology 19, 12 2021.
Dalzon, B., et al., Poly(Lactic Acid) Nanoparticles Targeting alpha5beta1 Integrin as Vaccine Delivery Vehicle, a Prospective Study. PLoS One, 2016. 11(12): p. e0167663.
